Adnan Halim
Associate Professor
Glycomics Program
Blegdamsvej 3, 2200 København N.
Member of:
Primary fields of research
Glycosylation is an essential cellular process that involves the covalent attachment of glycans (carbohydrates/sugars) to proteins; glycans play important roles in maintaining normal cellular functions and dysregulation of processes related to protein glycosylations are known to cause various diseases, e.g. cancer and developmental disorders. Our group is interested in the biosynthesis, regulation and biochemistry of O-linked glycosylations with a special focus on protein O-mannosylations (see our OA article in Cell). We use a combination of methods, including advanced mass spectrometry, to study structures, map site-specific locations and quantify changes of protein O-mannosylations on a proteome-wide scale. In addition, we use CRISPR/Cas9 gene editing for KO/KI of glycogenes in our efforts to study and understand enzymatic pathways involved in protein O-mannosylation.
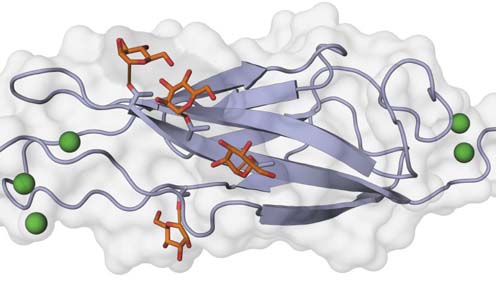
Cadherin O-mannosylation – the fourth extracellular cadherin (EC) domain of mouse E-cadherin is shown with O-linked mannose glycans (sticks) on specific β-strands. Calcium ions are depicted as green spheres. Illustration by Oliver Harrison, Julia Brasch and Lawrence Shapiro (Columbia University).
Current research
Cadherin-specific O-mannosylation by TMTC1-4
O-mannosylation was for a long time believed to be a rare type of protein modification in mammals. Until recently, α-dystroglycan (αDG), a component of the dystrophin complex, remained the only well-characterized protein with respect to O-mannosylation sites and structures. Building on the SimpleCell technology developed at Copenhagen Center for Glycomics (CCG), we established a glycoproteomic workflow for studying protein O-mannosylations on a proteome-wide scale. This approach allowed us to greatly expand the human O-mannose glycoproteome and led to the discovery of cadherins and protocadherins as major carriers of O-mannosylations. Using the SimpleCell technology, we recently uncovered that O-mannosylation of the cadherin superfamily is not mediated by the classical POMT1/POMT2 enzymes and identified the glycosyltransferase family (GT105), composed of TMTC1-4, that initiates the cadherin-specific O-mannosylation. Currently, we are characterizing the TMTC1-4 enzyme family, exploring the substrate specificities/interactomes of individual TMTC family members and studying the disease-causing mutations in this enzyme family. Our ambitions are to gain further understanding on cellular processes related to the function of cadherin-specific O-mannosylation in health and disease (e.g. Cobblestone lissencephaly).
Identification of new genes and pathways involved in O-mannosylation and cell-cell communication
In addition to the cadherin superfamily, we have identified plexins as a major class of cell-surface O-mannosylated proteins. The IPT/TIG domains of plexins are frequently O-mannosylated on specific β-strands but, intriguingly, this O-mannosylation doesn’t seem to be mediated by POMT1/POMT2 or the TMTC1-4 family. Our results thus indicate that other, as yet unknown glycosyltransferases are present in mammalian systems and are responsible for directing O-mannosylation specifically to proteins with IPT/TIG folds. Using the SimpleCell platform, we are currently screening candidate genes to test this hypothesis and to identify the novel glycosyltransferase enzymes responsible for O-mannosylation in mammalian cells.
O-Man glycoproteome discovery
The evolution of multiple O-glycosylation pathways was likely driven by a need to adapt higher organism to their environment and for their cells/proteins to obtain more specialized properties and functions, tunable via different types of O-glycans, to control e.g. protein trafficking, cell adhesion, protease cleavage and signaling. However, the precise roles of O-Man glycans on specific proteins are largely unknown, mainly due to a lack in the understanding of which proteins and sites are modified by O-Man glycans (the O-Man glycoproteome) and how this specificity is achieved.
Developing new techniques capable of decoding the regulations and functions of O-Man glycoproteomes and may thus lead to the next breakthrough in the understanding of how cellular functions are regulated and fine-tuned by O-glycans, how O-Man glycoproteomes contribute to health and disease, and ultimately, how this important class of molecules could be exploited for development of new strategies for diagnostics, drugs and treatments.
In this project, we develop sensitive glycopeptide enrichment protocols for site-specific, quantitative O-Man glycoproteomics in cell models and tissues. Specific applications include O-Man discovery in model organisms, e.g. Drosophila melanogaster and mice (brain).
O-Man dependent interactomics
Adhesion molecules and receptors engage in complex physical interactions that rely on extensive protein-protein interaction networks. However, insight into how the novel O-Man glycans on distinct protein domains influence cadherin and plexin receptor interaction is completely lacking. Testing specific hypotheses for glycoproteins in general has remained challenging, and while great progress has been made towards understanding direct glycan-protein binding, far less is known about how glycosylation status diversifies protein-protein interactions (interactomes).
We aim to develop novel analytical platforms integrating precise gene editing, endogenous tagging, glycoproteomics and interactomics to address an important unmet need for new, innovative solutions capable of resolving glycoprotein interactomes in the context of their (differential) glycosylation status.
The mucin-like KIAA1549 protein
Genetic defects involving the KIAA1549 gene underlie human disorders, including Pilocytic Astrocytoma (PA), which accounts for the most common form of pediatric brain tumors. The most common genetic alternation in PA results in a KIAA1549-BRAF kinase fusion protein with constitutive BRAF kinase activity which impacts the MAPK/RAS signaling pathway. However, the molecular functions of KIAA1549 remain unknown, and this knowledge gap hampers further advances in the understanding of molecular mechanisms in pediatric cancers.
We previously discovered that KIAA1549 is a heavily O-Man glycosylated protein and a target for the POMT1/POMT2 pathway. The overarching objective in this project is to apply genetic engineering, mass spectrometry and cell models to characterize the O-Man glycosylation profile and molecular functions of KIAA1549 in health and disease.
Collaborators
- John LaCava (The Rockefeller University, NY, USA)
- Thomas Worzfeld (University of Marburg, Germany)
- Vladislav Panin (Texas A&M University, USA)
- Joseph Gleeson (University of California San Diego, USA)
ID: 40443472
Most downloads
-
279
downloads
Multiple distinct O-Mannosylation pathways in eukaryotes
Research output: Contribution to journal › Review › Research › peer-review
Published -
238
downloads
Mapping the O-Mannose Glycoproteome in Saccharomyces cerevisiae
Research output: Contribution to journal › Journal article › Research › peer-review
Published -
79
downloads
Display of the human mucinome with defined O-glycans by gene engineered cells
Research output: Contribution to journal › Journal article › Research › peer-review
Published
